Within the vast expanse of the universe lie elements that are exceedingly rare, elusive, and intriguing. From radioactive isotopes to fleeting traces found in ores, these elements capture the imagination of scientists and enthusiasts alike. In this exploration, we delve into the realm of the rarest elements in the universe, each with its own unique properties and origins. From the scarcely seen astatine to the elusive promethium, we uncover the mysteries behind these rare elements, shedding light on their atomic makeup, scarcity, and potential applications. Join us on a journey to discover the hidden treasures of the periodic table and unravel the secrets of the rarest elements in existence.
Astatine (At)
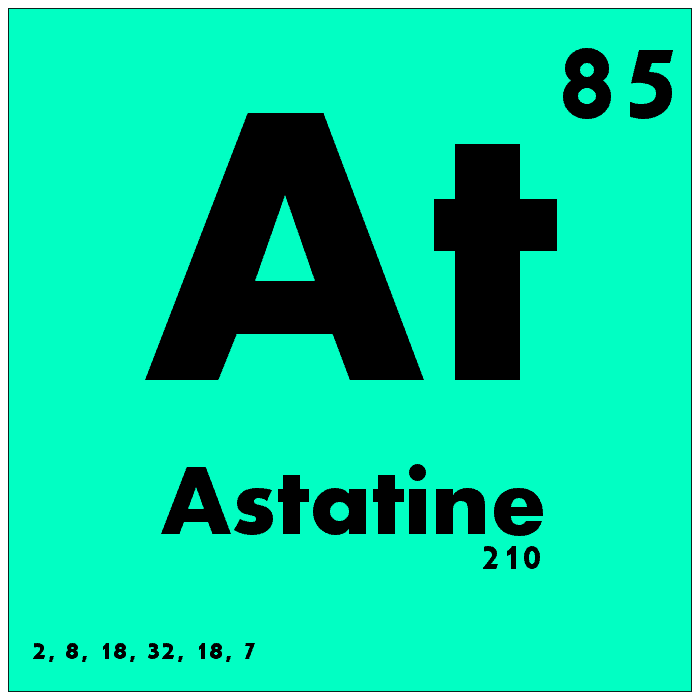
Astatine is one of the rarest naturally occurring elements in the universe, with its presence limited to trace amounts in uranium and thorium ores. It is highly radioactive and has no stable isotopes, making it extremely rare and difficult to study. Astatine’s atomic number is 85, and its atomic mass varies depending on the isotope. Due to its extreme rarity and high radioactivity, astatine has very few practical uses, but it has been studied for its potential applications in nuclear medicine and cancer treatment.
Promethium (Pm)
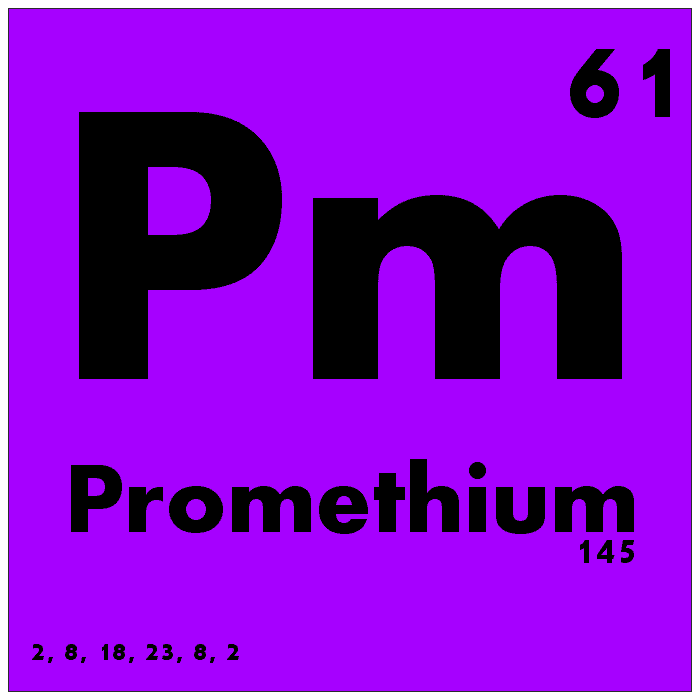
Promethium is one of the rarest and most elusive elements in the universe, with no stable isotopes and very few naturally occurring isotopes. It is typically found in trace amounts in uranium ores but is primarily produced artificially in nuclear reactors. Promethium’s atomic number is 61, and its atomic mass is approximately 145. Promethium has very few practical applications due to its scarcity and radioactivity, but it has been used in specialized nuclear batteries and luminous paints.
Francium (Fr)

Francium is one of the rarest naturally occurring elements in the universe, with its presence limited to trace amounts in uranium and thorium ores. It is the second rarest naturally occurring element, after astatine. Francium’s atomic number is 87, and its atomic mass is approximately 223. Due to its extreme rarity and high radioactivity, francium has very few practical uses and is primarily studied for research purposes.
Technetium (Tc)

Technetium is one of the rarest naturally occurring elements in the universe, with no stable isotopes and very few naturally occurring isotopes. It is typically produced artificially in nuclear reactors or particle accelerators. Technetium’s atomic number is 43, and its atomic mass is approximately 98. Technetium has limited practical applications but is used in nuclear medicine for diagnostic imaging and cancer treatment.
Protactinium (Pa)
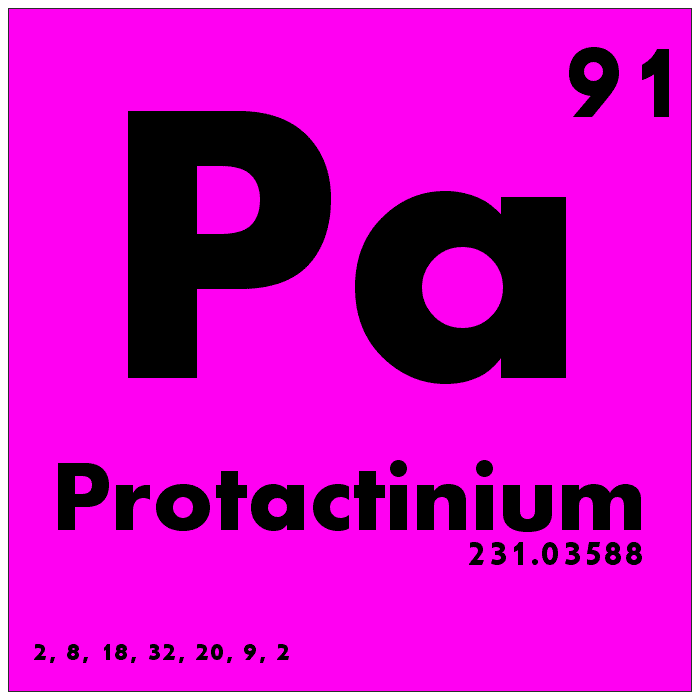
Protactinium is one of the rarest naturally occurring elements in the universe, with its presence limited to trace amounts in uranium ores. It is highly radioactive and has no stable isotopes, making it extremely rare and difficult to study. Protactinium’s atomic number is 91, and its atomic mass is approximately 231. Protactinium has very few practical uses due to its scarcity and radioactivity, but it has been studied for its potential applications in nuclear reactors and weapons.
Polonium (Po)
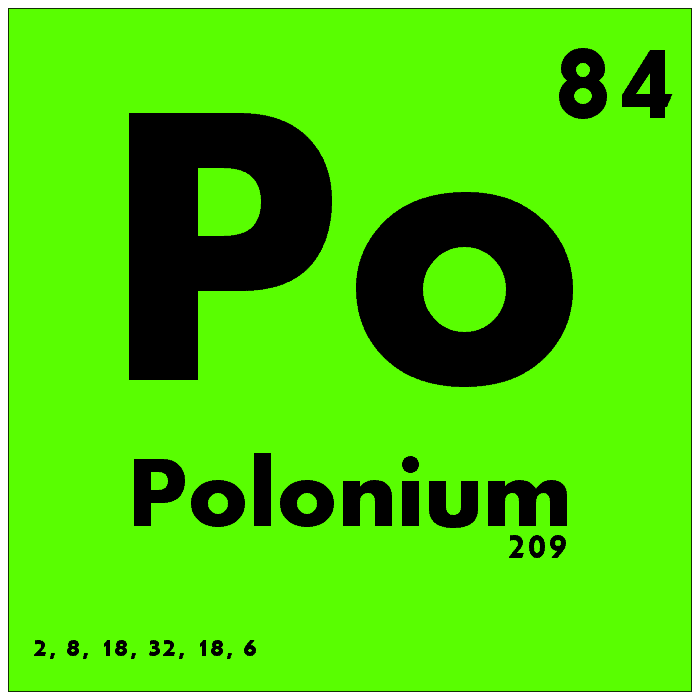
Polonium is one of the rarest naturally occurring elements in the universe, with its presence limited to trace amounts in uranium ores. It is highly radioactive and has no stable isotopes, making it extremely rare and difficult to study. Polonium’s atomic number is 84, and its atomic mass is approximately 210. Polonium has very few practical uses due to its extreme toxicity and radioactivity, but it has been used in nuclear reactors and weapons.
Radium (Ra)

Radium is one of the rarest naturally occurring elements in the universe, with its presence limited to trace amounts in uranium and thorium ores. It is highly radioactive and has no stable isotopes, making it extremely rare and difficult to study. Radium’s atomic number is 88, and its atomic mass is approximately 226. Radium has very few practical uses due to its extreme toxicity and radioactivity, but it has been used in luminous paints and medical treatments.
Neptunium (Np)

Neptunium is one of the rarest naturally occurring elements in the universe, with no stable isotopes and very few naturally occurring isotopes. It is typically produced artificially in nuclear reactors or particle accelerators. Neptunium’s atomic number is 93, and its atomic mass is approximately 237. Neptunium has very few practical applications but has been used in nuclear reactors and weapons research.
Americium (Am)

Americium is one of the rarest naturally occurring elements in the universe, with no stable isotopes and very few naturally occurring isotopes. It is typically produced artificially in nuclear reactors or particle accelerators. Americium’s atomic number is 95, and its atomic mass is approximately 243. Americium has limited practical applications but has been used in smoke detectors and medical devices.
Curium (Cm)
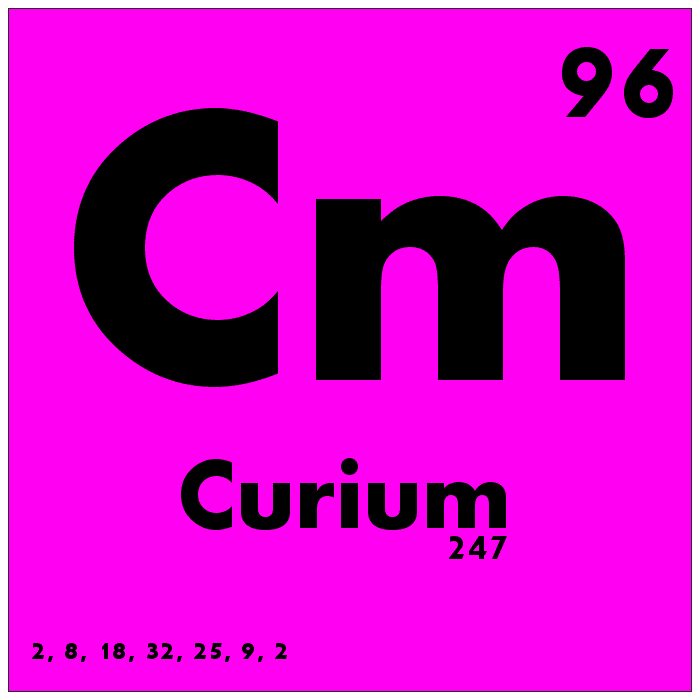
Curium is one of the rarest naturally occurring elements in the universe, with no stable isotopes and very few naturally occurring isotopes. It is typically produced artificially in nuclear reactors or particle accelerators. Curium’s atomic number is 96, and its atomic mass is approximately 247. Curium has very few practical applications but has been used in research and as a heat source for spacecraft.
This article originally appeared on Rarest.org.
